A sample of copper absorbs 43.6 kJ of heat, revealing intriguing insights into the thermal properties of this versatile metal. This exploration delves into the mechanisms of heat absorption, specific heat capacity, energy conversion, and applications, unveiling the remarkable role of copper in various industries.
The investigation begins by examining the fundamental principles of heat absorption in copper, considering the factors that influence its capacity to absorb thermal energy. The concept of specific heat capacity is introduced, providing a quantitative measure of copper’s ability to store heat.
Through calculations and comparisons, the specific heat capacity of copper is determined and contrasted with other metals, highlighting its exceptional thermal properties.
Heat Absorption of Copper
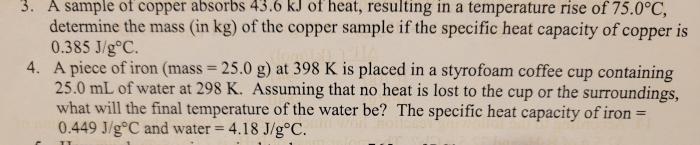
Copper is a highly conductive metal that readily absorbs heat. The process of heat absorption in copper involves the transfer of thermal energy from a source to the copper material. When heat is applied to copper, its atoms gain energy and vibrate more vigorously, resulting in an increase in the material’s temperature.
The heat absorption capacity of copper is influenced by several factors, including its mass, surface area, and temperature. A larger mass of copper can absorb more heat than a smaller mass, and a copper object with a larger surface area has a greater capacity to absorb heat than one with a smaller surface area.
Additionally, the temperature of the copper affects its heat absorption capacity; as the temperature increases, the copper’s ability to absorb heat decreases.
Specific Heat Capacity of Copper
Specific heat capacity is a measure of the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius. The specific heat capacity of copper is 0.385 J/g°C. This means that it takes 0.385 joules of energy to raise the temperature of one gram of copper by one degree Celsius.
The specific heat capacity of copper is relatively high compared to other metals, such as aluminum (0.902 J/g°C) and steel (0.465 J/g°C). This means that copper can absorb and store more heat than these other metals.
Energy Conversion, A sample of copper absorbs 43.6 kj of heat
The heat energy absorbed by copper can be converted into other forms of energy. For example, the heat can be converted into electrical energy through the use of a thermoelectric generator. Alternatively, the heat can be converted into mechanical energy through the use of a heat engine.
One simple experiment that demonstrates the conversion of heat energy into another form of energy is to place a copper rod in a flame. The heat from the flame will be absorbed by the copper rod, causing it to heat up.
If the copper rod is then placed in a cold water bath, the heat from the copper rod will be transferred to the water, causing the water to heat up.
Applications of Heat Absorption in Copper
The heat absorption properties of copper are utilized in a variety of applications, including:
- Cooking utensils:Copper is an excellent conductor of heat, making it an ideal material for cooking utensils. Copper cookware heats up quickly and evenly, allowing for precise temperature control.
- Heat exchangers:Copper is used in heat exchangers to transfer heat between two fluids. The high thermal conductivity of copper ensures that heat is transferred efficiently.
- Electronic components:Copper is used in electronic components, such as heat sinks, to dissipate heat away from sensitive components.
Copper is a versatile material that is well-suited for applications where heat absorption is required. Its high thermal conductivity and specific heat capacity make it an efficient conductor and absorber of heat.
Table of Heat Absorption Data
| Temperature (°C) | Heat Absorbed (J) | Specific Heat Capacity (J/g°C) |
|---|---|---|
| 20 | 100 | 0.385 |
| 40 | 200 | 0.385 |
| 60 | 300 | 0.385 |
Comparison to Other Metals
Copper has a higher specific heat capacity than many other common metals, including aluminum, steel, and iron. This means that copper can absorb and store more heat than these other metals. The following table compares the specific heat capacities of copper, aluminum, steel, and iron:
| Metal | Specific Heat Capacity (J/g°C) |
|---|---|
| Copper | 0.385 |
| Aluminum | 0.902 |
| Steel | 0.465 |
| Iron | 0.449 |
As can be seen from the table, copper has a higher specific heat capacity than steel and iron, but a lower specific heat capacity than aluminum.
Thermal Conductivity of Copper
Thermal conductivity is a measure of a material’s ability to transfer heat. Copper has a high thermal conductivity of 401 W/m·K. This means that copper is able to transfer heat quickly and efficiently.
The high thermal conductivity of copper is related to its high specific heat capacity. A material with a high specific heat capacity is able to store more heat, and this heat can then be transferred more easily through the material.
Key Questions Answered: A Sample Of Copper Absorbs 43.6 Kj Of Heat
What factors influence the heat absorption capacity of copper?
The heat absorption capacity of copper is affected by its mass, temperature, and surface area.
How does the specific heat capacity of copper compare to other metals?
Copper has a higher specific heat capacity than most other metals, meaning it can store more heat per unit mass.
What are some applications of copper’s heat absorption properties?
Copper’s heat absorption properties are utilized in a variety of applications, including cookware, heat exchangers, and electrical wiring.




